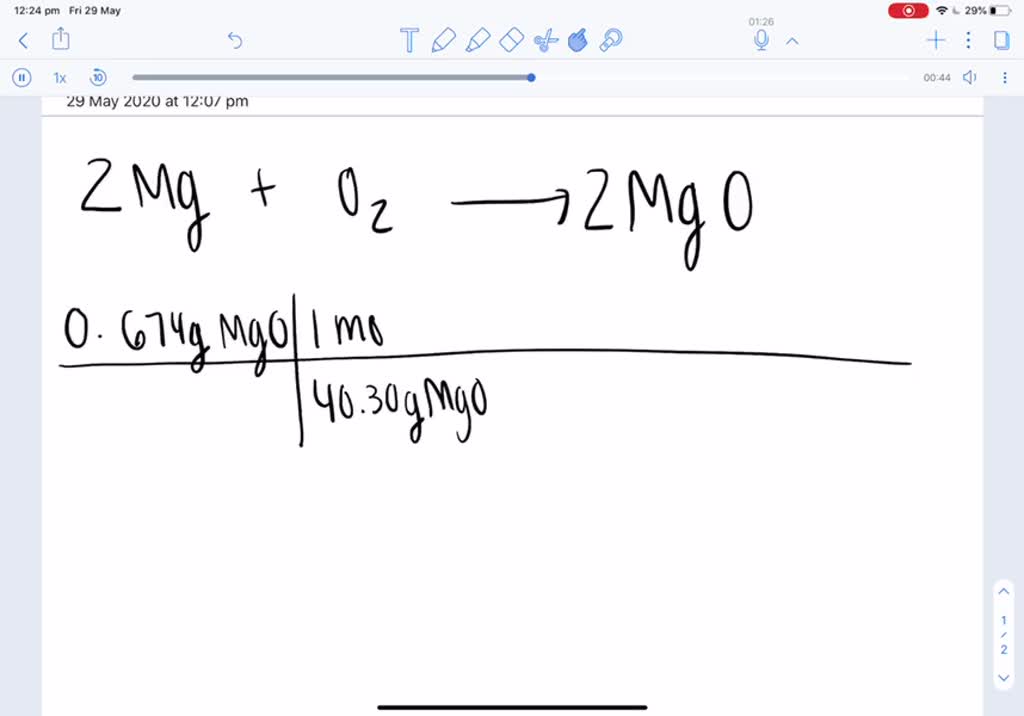
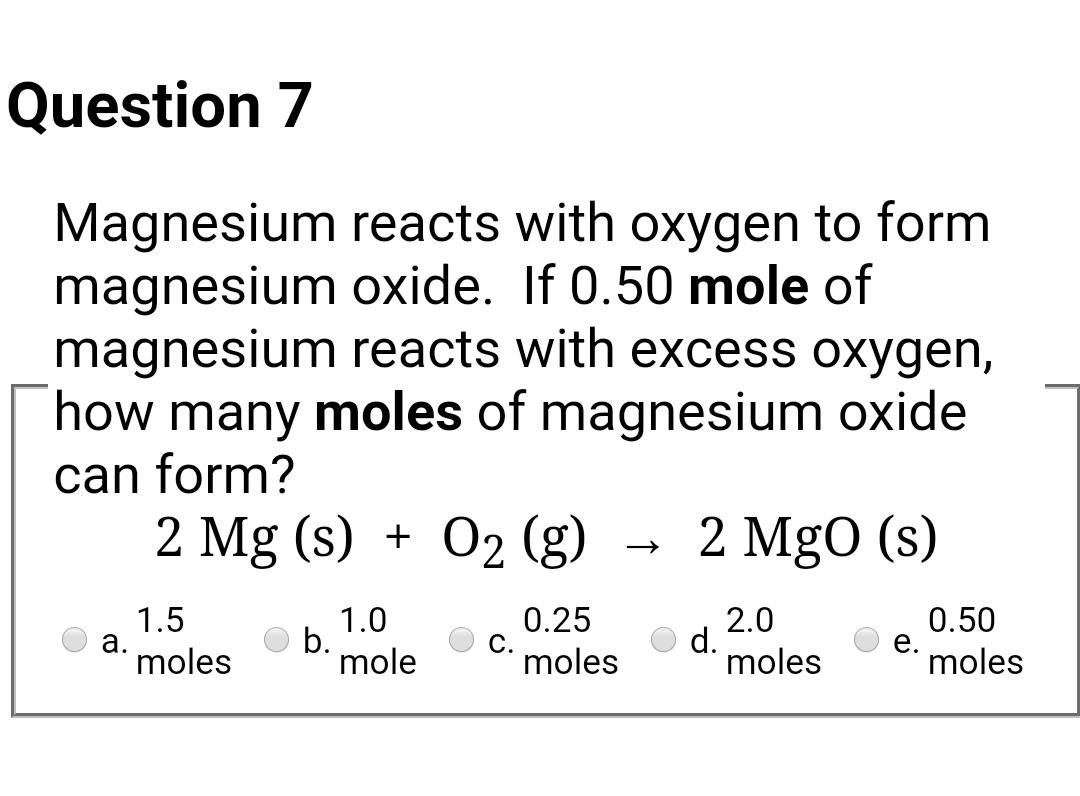
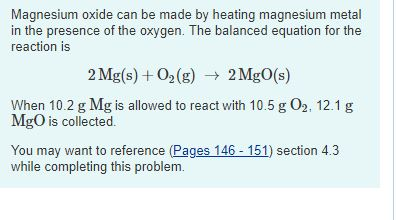SOLVED: A 0.406 g sample of magnesium reacts with oxygen, producing 0.674 g of magnesium oxide as the only product. What mass of oxygen was consumed in the reaction?
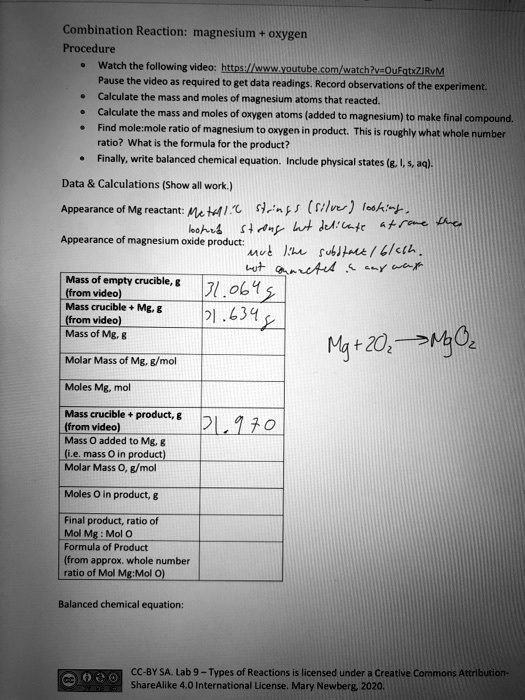
SOLVED: Combination Reaction; Magnesium Oxygen Procedure Watch the following video: https://www.youtube.com/watch?yaQuEgtxZiivM Pause the video to get data readings. Record observations of the experiment. Calculate the mass and moles of magnesium atoms ...
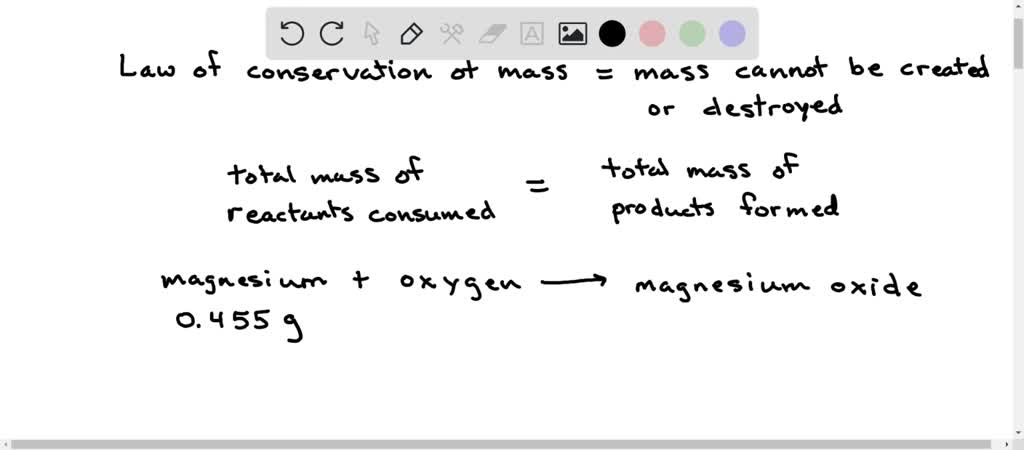
SOLVED: (Law of Conservation of Mass) A 0.455 g sample of magnesium is allowed to burn in 2.315 g of oxygen gas. The sole product is magnesium oxide. After the reaction, no

Amazon.com: Wonder Labs Magnesium Oxide 400, 483mg of Magnesium Oxide Pharmaceutical Grade** Compare to MAG-OX 400 ® - 250 Tablets : Health & Household