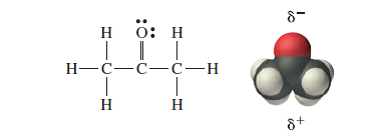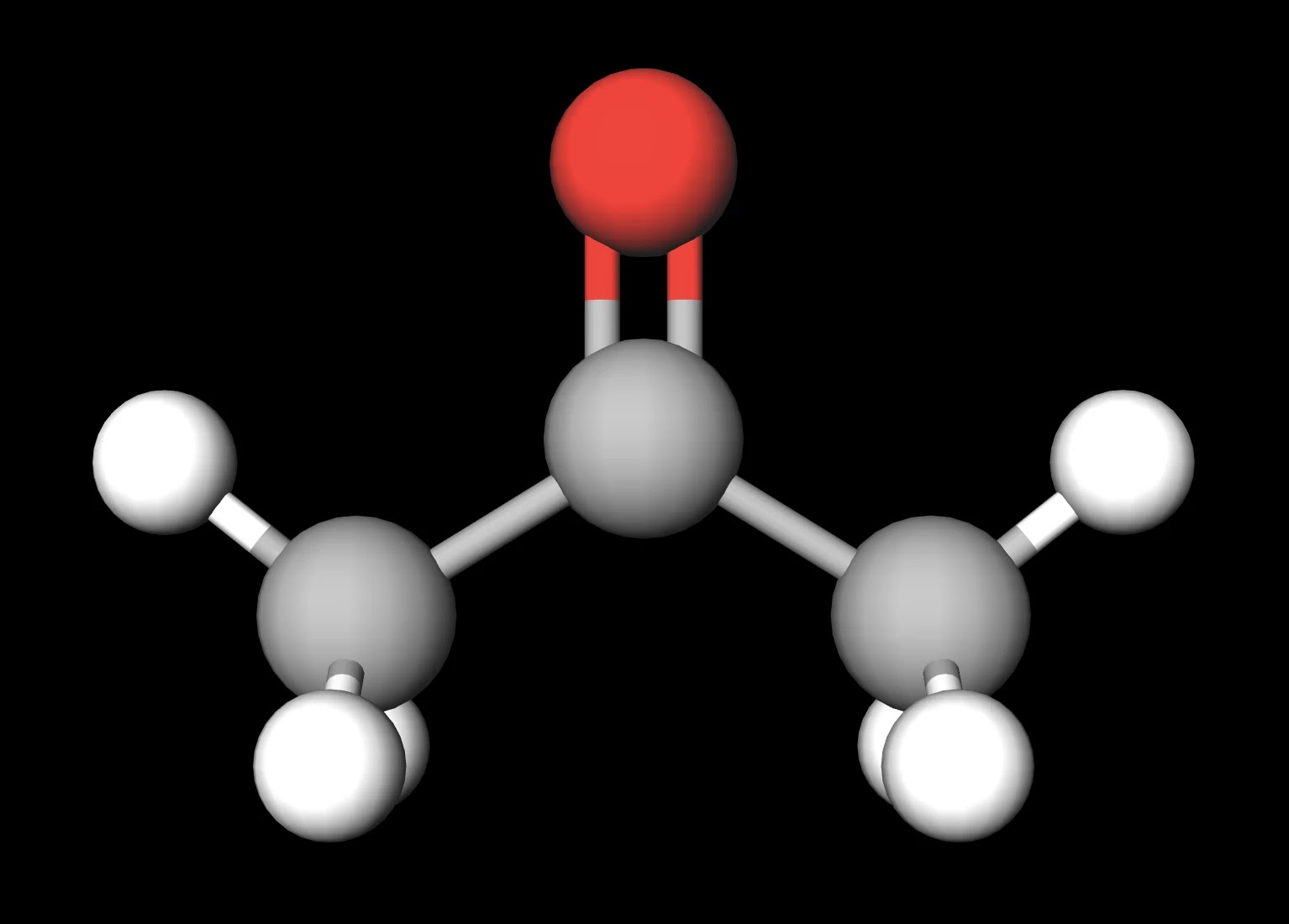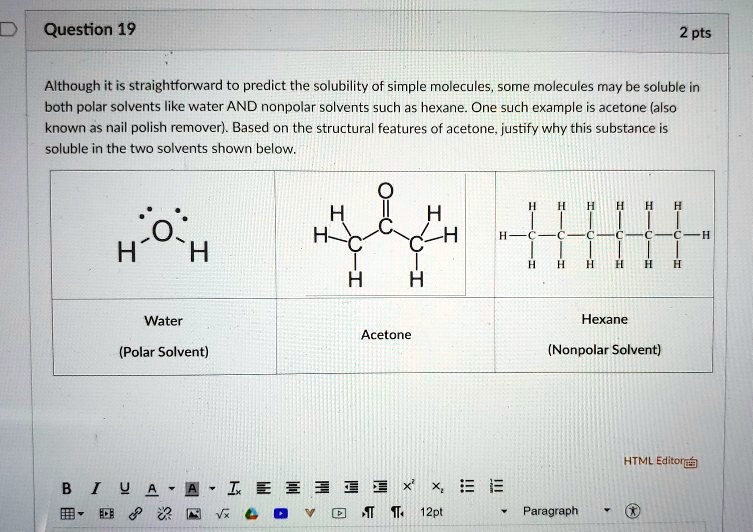
SOLVED: Question 19 2 pts Although it is straightforward to predict the solubility of simple molecules some molecules may be soluble in both polar solvents like water AND nonpolar solvents such as

filosoffen.dk - what is metformin 500 mg used for | Is acetone polar or nonpolar molecule are mistaken

polarity - Why does acetone have a greater dipole moment than dimethyl ether? - Chemistry Stack Exchange

Draw the structures of ethanol, acetone, toluene, hexane, and water. Classify each solvent as polar, nonpolar, or moderately polar. | Homework.Study.com
What makes acetone a really good solvent? What allows it to dissolve both polar and non-polar molecules? - Quora






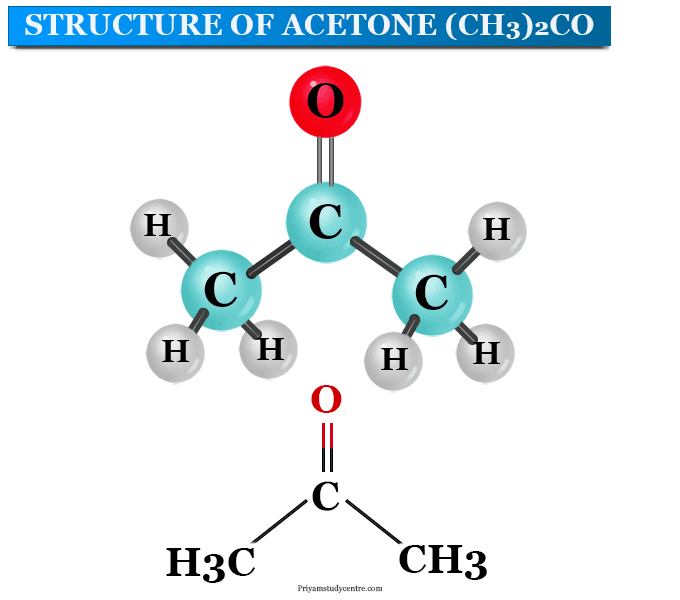

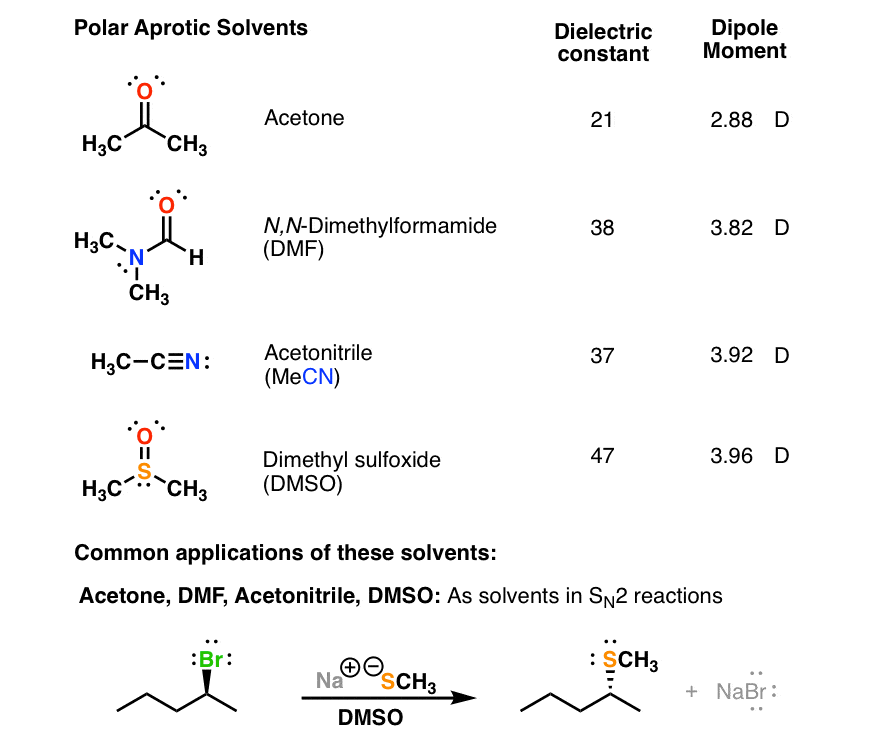




![Polarity values (literature data according to Ref. [18]). | Download Table Polarity values (literature data according to Ref. [18]). | Download Table](https://www.researchgate.net/publication/38014625/figure/tbl2/AS:668899632742421@1536489508874/Polarity-values-literature-data-according-to-Ref-18.png)