Establishing an FDA office in the Middle East/North Africa: An Abraham Accords Initiative - Food and Drug Law Institute (FDLI)

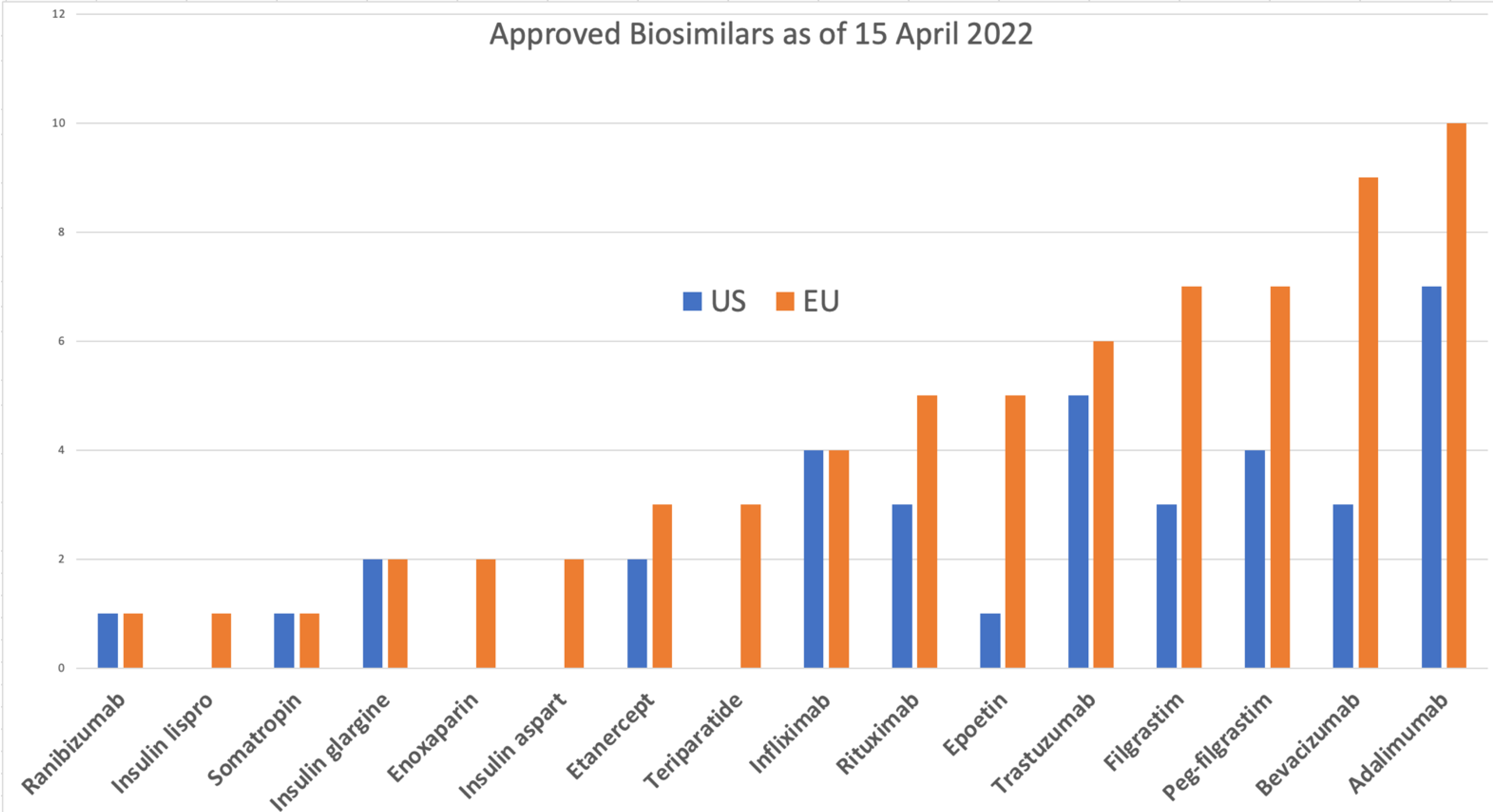
FDA's Interpretation Of The “Deemed To Be A License” Provision Of The Biologics Price Competition An | Contract Pharma
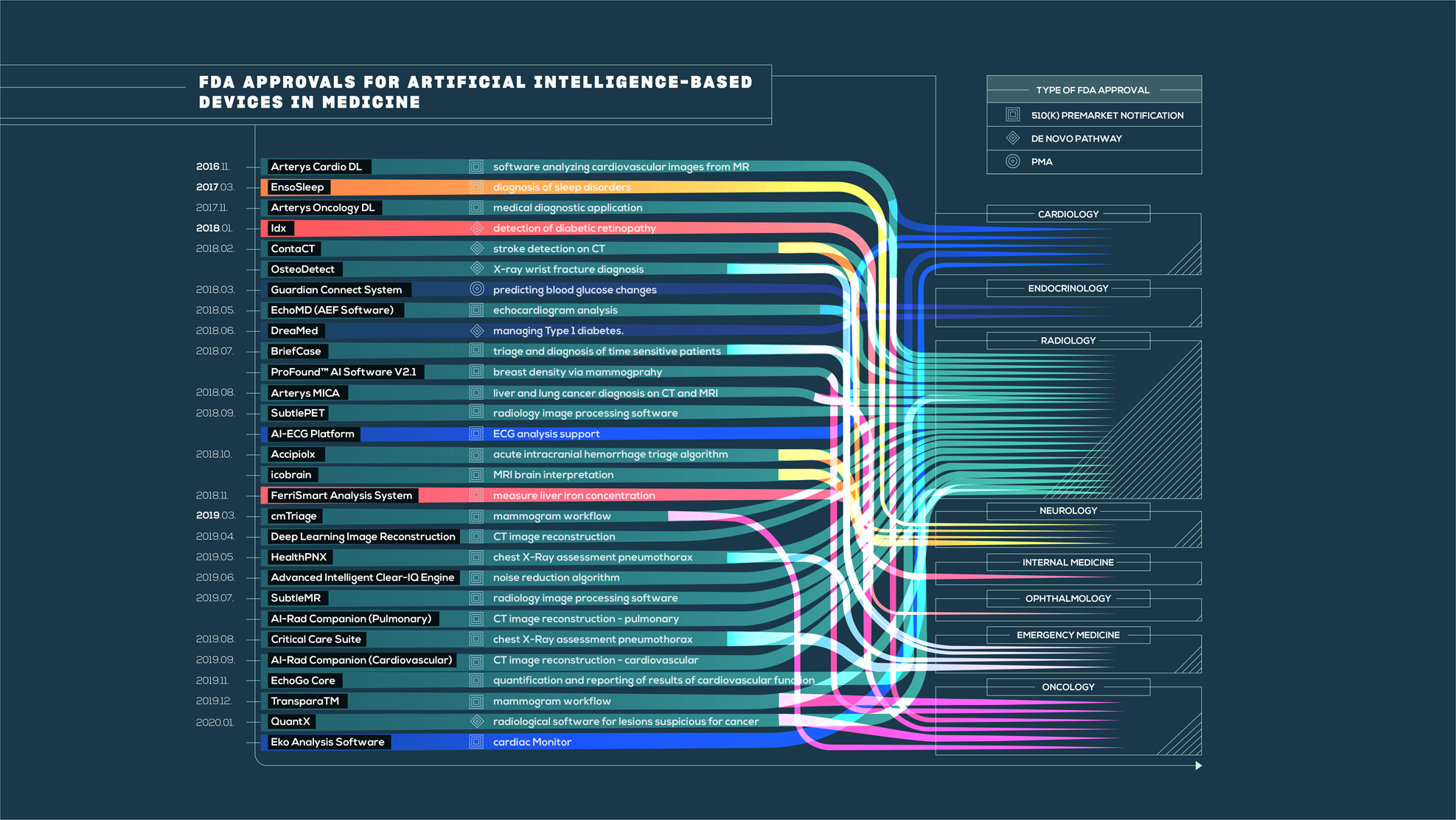
The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database | npj Digital Medicine
Foreign Supplier Verification Programs (FSVP) Importer Portal for FSVP Records Submission User Guide

Meeting FDA Guidance recommendations for replication-competent virus and insertional oncogenesis testing: Molecular Therapy - Methods & Clinical Development
FDA Food Facility Registration is required under laws created by both the Bioterrorism Act of 2003 and Food Safety Modernization

The FDA Verification Portal 🔎 Check if an establishment is licensed and/or verify if a health product is registered with the Food and Drug... | By Food and Drug Administration Philippines

Structural Alert/Reactive Metabolite Concept as Applied in Medicinal Chemistry to Mitigate the Risk of Idiosyncratic Drug Toxicity: A Perspective Based on the Critical Examination of Trends in the Top 200 Drugs Marketed